Basics of the Process: Single Stage Distillation 101

Over the years we have encountered operators and supervisors who knew how their recovery system functioned and how to respond if they received an alarm signal on their system control panel, however these same folks did not understand the basic single stage distillation concept or what to do if something changed in the makeup of their process waste solvent feed-stock. This may be old hat to some of you but please bear with us and we cover these basics. This post will lay a foundation for future entries into answering these questions.
How It Works:
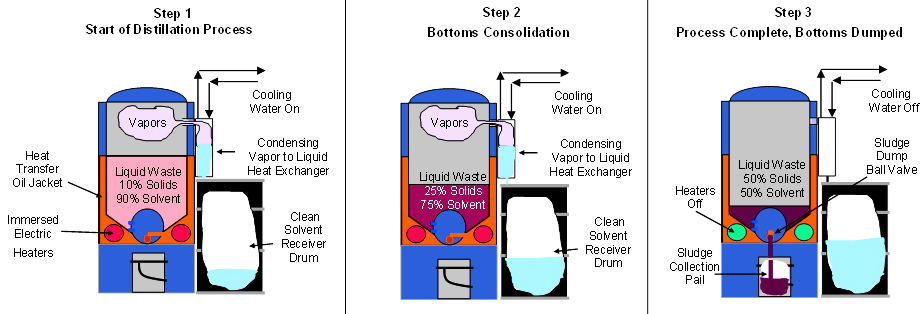
Solvent recycling is accomplished by a distillation process that includes the following steps:
- A distillation vessel (still) processes spent waste solvent solution on a batch or continuous basis.
- A thermal oil jacket, electrically or steam heated, injects heat into the waste solvent by conductive thermal transfer.
- The still may be operated under vacuum, which lowers the boiling temperature of the solvent
- When the waste solution reaches its boiling point, the solvent changes from liquid to a vapor (gas) at a controlled rate.
- The clean solvent, in a vapor phase, passes through the condenser which has both a condensing and a sub-cooling section. In the condenser, the solvent changes back to a liquid and is cooled back to ambient temperature
- The contaminants (solids or non-volatile liquids) do not undergo a vapor phase, but stay behind as “still bottoms” to be discharged out the drain port.
Once the non-volatile portion is build up to concentrated levels, the “still bottoms” are discharged. Another production run can then be initiated. Below is a basic illustration of the process.
Categories & Tags
ISO 9001:2015
